This video explains how sepsis induced by an overload of blood pathogens can be treated with the Wyss Institute’s improved pathogen-extracting, spleen-mimicking device. Blood is flown through a cartridge filled with hollow fibers that are coated with a genetically engineered blood protein inspired by a naturally-occurring human molecule called Mannose Binding Lectin (MBL). MBL is found in our innate immune
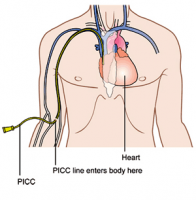 PICC line catheters are not necessarily better than other central venous catheters, but they may affect the patient’s activities more than other types of catheters. But tunneled catheters and implanted ports are the other types of central venous catheters, which are tubes threaded through veins to a spot near the heart for the purposes of giving medications, blood or kidney dialysis. Tunneled catheters and implanted ports are typically left under the skin to keep them in place and require less care than a PICC line, PICC lines are central catheters inserted into a vein in the arm, whereas other catheters are placed in the neck or chest.
PICC line catheters are not necessarily better than other central venous catheters, but they may affect the patient’s activities more than other types of catheters. But tunneled catheters and implanted ports are the other types of central venous catheters, which are tubes threaded through veins to a spot near the heart for the purposes of giving medications, blood or kidney dialysis. Tunneled catheters and implanted ports are typically left under the skin to keep them in place and require less care than a PICC line, PICC lines are central catheters inserted into a vein in the arm, whereas other catheters are placed in the neck or chest.
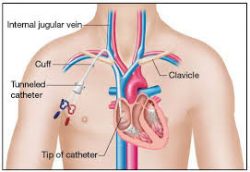 Long-term central venous access devices, external catheters, and implanted ports have found
Long-term central venous access devices, external catheters, and implanted ports have found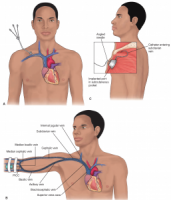 great use for administration of inpatient and outpatient, for intravenous antibiotics and for intravenous nutrition. Maintaining a clean PICC line area is important for preventing complications such as infections, and keeping careful watch over the line insertion is critical, a person with a PICC line needs to watch for changes in skin color, and report changes immediately to a medical professional. For preventative care, it is important to wash hands frequently and avoid touching the catheter or dressing.
great use for administration of inpatient and outpatient, for intravenous antibiotics and for intravenous nutrition. Maintaining a clean PICC line area is important for preventing complications such as infections, and keeping careful watch over the line insertion is critical, a person with a PICC line needs to watch for changes in skin color, and report changes immediately to a medical professional. For preventative care, it is important to wash hands frequently and avoid touching the catheter or dressing.
The International Gastrointestinal Eosinophilic Researchers (TIGER) are a group of pediatric and adult physicians and basic scientists interested in understanding the underlying science and clinical effects that eosinophils have on disease. TIGER currently includes physicians from the specialties of gastroenterology, allergy, and pathology as well as researchers who are dedicated to conducting laboratory, clinical and genetic research on eosinophilic and allergic disorders of the gastrointestinal tract. The group also works with medical societies and community advocacy groups to educate providers and the public.
Not Just for Adults Anymore. Doctor, Evan S Dellon
(This is very long I have only taken a wee bit of this study follow the link to download the PDF yourself)
RE. Dilation of pediatric eosinophilic esophagitis: adverse events and short-term outcomes
Eosinophilic esophagitis (EoE) is a chronic immune/ antigen-mediated disorder characterized by eosinophilic infiltration of the esophageal mucosa (J Allergy Clin Immunol 2011;128:3–20; Gastroenterology 2015;148: 1143–1157). The chronic esophageal inflammation increases the risk of developing fibrosis, esophageal strictures, and long-segment narrowing, but this has largely been noted in adult patients (Gastrointest Endosc 2014;79: 577–585). When these complications occur, esophageal dilation may be necessary. Although there was originally a concern that this procedure held significant risks in EoE, recent studies primarily examining adult patients demonstrate that the risk of serious complications such as perforation is low and that dilation can be safely performed (Am J Gastroenterol 2016;111:206–213; Gastrointest Endosc 2017;86:581–591.e3). However, there are limited data regarding safety and tolerance outcomes in children with EoE who require esophageal dilation. The study by Menard-Katcher et al is one of the first to assess the adverse event rate and short-term outcomes of November 2017 Selected Summaries 1445 esophageal dilation in children. They aimed to evaluate differences in dilation-related complications between patients with EoE and without EoE as well as those undergoing standard upper endoscopy. This study is a retrospective assessment of children 18 years and younger at a tertiary academic medical center over a 5- year period. Of the 451 total dilations identified, 68 were performed in 40 EoE patients. Of the EoE patients, 43% required a repeat dilation during the study period. Non– wire-guided bougies (Maloney dilators) were used for the majority of the procedures (72%), with the remainder completed with through-the-scope balloons. There were no major complications, such as perforations or significant hemorrhage. Chest pain was reported after 14.7% of EoE dilations. Dilation-related adverse events rates of grade 2 or higher (those requiring unanticipated medical intervention) were seen in 2.9% of EoE and 3.1% of non-EoE patients (P > .5). In the EoE patients, the 2 adverse events were post-dilation chest pain requiring chest radiographs and chest pain requiring overnight observation for analgesia
http://www.gastrojournal.org/article/S0016-5085(17)36195-4/abstract
2015 Apr
Treatment outcomes for eosinophilic esophagitis in children with esophageal atresia. EoE
Eosinophilic esophagitis (EoE) has been reported to be more prevalent in patients with esophageal atresia/tracheoesophageal fistula (EA-TEF). To date, there is limited data on the management of EoE in this group of patients. The aim of this study is to evaluate the treatment outcomes of EoE in children with EA-TEF. A retrospective chart review was performed on all EA-TEF children who were diagnosed with and treated for EoE between January 2000 and September 2013 at the Sydney Children’s Hospital. Data collected included details of the patient’s treatment, post-treatment endoscopy, symptoms, and nutrition. Twenty patients were included in the study. Median age at diagnosis was 26 months (8-103 months), and median time from diagnosis to the last follow-up was 23 months (2-132 months). Patients were treated with budesonide slurry, swallowed fluticasone, elimination diet alone or in combination. All patients were on proton pump inhibitors at the time of diagnosis of EoE which was continued. Six out of seven patients who had furrowing/exudate in endoscopy at diagnosis had a complete resolution at a median follow-up period of 26 months (P = 0.031). Median peak intraepithelial eosinophil count reduced significantly from 30/high-powered field (HPF) (19-80/HPF) to 8/HPF (0-85/HPF) (median time for improvement = 24 months) (P = 0.015). There was a significant reduction in symptoms of dysphagia and reflux post-treatment (P < 0.001). Prevalence of strictures significantly decreased (P = 0.016), as did need for dilatations (P = 0.004). In four out of six patients with gastrostomies at baseline, the feeding improved on the treatment of EoE and the gastrostomy could be closed. There was also a nonsignificant trend towards improvement in weight and height ‘z scores’ of the patients. Treatment of EoE in children with EA-TEF was found to significantly reduce intraepithelial eosinophil count, symptoms, strictures and need for dilatations.
www.ncbi.nlm.nih.gov/pubmed/25872589
Eosinophilic Esophagitis (EoE disease) in Children
Eosinophilic esophagitis (EoE) is an inflammatory condition in which the wall of the esophagus becomes filled with large numbers of white blood cells called eosinophils.Because this condition inflames the esophagus, someone with EoE may experience difficulty swallowing, pain, nausea, regurgitation, and vomiting. Over time, the disease can cause the esophagus to narrow, which sometimes results in food becoming stuck, or impacted, within the esophagus, requiring emergency removal.In young children, many of the symptoms of eosinophilic esophagitis resemble those of gastroesophageal reflux disease (GERD)—including feeding disorders and poor weight gain—so the child may be mistakenly diagnosed with GERD. However, proper diagnosis of esophagitis in children is important because it is a serious disease that can cause lifelong problems if undiagnosed.
Quick Facts about Eosinophilic Esophagitis
- 75% of individuals with EoE are white males
- EoE occurs in approximately 1 in 10,000 people
- The exact cause of EoE is unknown, but it appears to be related to food allergies
- EoE is more common in patients with other allergic diseases, such as asthma
- EoE was discovered relatively recently and much remains unknown about the disease
- In 2006, CDHNF and other experts formed The International Gastrointestinal Eosinophil Researchers (TIGER) to study the role of eosinophils in gastrointestinal diseases.
2014 Dec
Eosinophilic esophagitis in patients with esophageal atresia and chronic dysphagia.
Esophageal atresia (EA) is defined as a discontinuity of the lumen of the esophagus repaired soon after birth. Dysphagia is a common symptom in these patients, usually related to stricture, dysmotility or peptic esophagitis. We present 4 cases of patients with EA who complained of dysphagia and the diagnosis of Eosinophilic esophagitis (EoE) was made, ages ranging from 9 to 16 years. Although our patients were on acid suppression years after their EA repair, they presented with acute worsening of dysphagia. Esophogastro duodenoscopy and/or barium swallow did not show stricture and biopsies revealed elevated eosinophil counts consistent with EoE. Two of 4 patients improved symptomatically with the topical steroids. It is important to note that all our patients have asthma and 3 out of 4 have tested positive for food allergies. One of our patients developed recurrent anastomotic strictures that improved with the treatment of the EoE. A previous case report linked the recurrence of esophageal strictures in patients with EA repair with EoE. Once the EoE was treated the strictures resolved. On the other hand, based on our observation, EoE could be present in patients without recurrent anastomotic strictures. There appears to be a spectrum in the disease process. We are suggesting that EoE is a frequent concomitant problem in patients with the history of congenital esophageal deformities, and for this reason, any of these patients with refractory reflux symptoms or dysphagia (with or without anastomotic stricture) may benefit from an endoscopic evaluation with biopsies to rule out EoE.
www.ncbi.nlm.nih.gov/pmc/articles/PMC4273156/
eosinophilic esophagitis: symptoms & diagnosis
Does My Child Have Eosinophilic Esophagitis?
Eosinophilic Esophagitis has only recently been identified as a disease, and many of its symptoms—particularly in children—mimic the symptoms of GERD.
Eosinophilic Esophagitis symptoms may include:
- Nausea
- Problems swallowing (dysphagia)
- Vomiting
- Stomach pain
- Chest pain
- Heartburn
- Loss of weight
- Food impaction
Patients of different ages tend to experience different symptoms:
- In children, Eosinophilic Esophagitis symptoms are usually similar to those of GERD (abdominal pain, nausea, vomiting, poor weight gain)
- Adolescents and adults frequently experience difficulty swallowing as well as food impaction
Reflux & GERD
www.gikids.org/files/multimedia/MedicalAnimationReflux.swf
The Difference Between Reflux and GERD in Kids
In babies, it’s called spitting up. In older kids, the signs of reflux and GERD can be burping, stomach aches, and heartburn. Most people experience acid reflux sometimes, and it’s usually not a problem. Even infants who spit up frequently are usually perfectly healthy.However, in some people, reflux happens so frequently and is so severe that it develops into a condition called gastroesophageal reflux disease (GERD). GERD occurs when reflux causes troublesome symptoms or complications such as failure to gain weight, bleeding, respiratory problems or esophagitis.You can develop GERD at any age. There are some differences between the symptoms, management, and treatment of GERD in infants and GERD in older children and teens. GIKids has resources and information about pediatric GERD, whether you have an infant, older child, or if you are a teen with GERD. The most important thing to know is that, with proper treatment, kids with GERD can lead normal, active lives.
Quick Facts about Reflux and GERD
- In many cases, GERD in kids can be managed with lifestyle changes, and without medication, GERD often runs in families
- Kids with GERD may have frequent complaints of abdominal pain or a tummy ache
- Children and teens with asthma are more likely to have GERD
Diagnosing Eosinophilic Esophagitis:
If your doctor suspects that your child has Eosinophilic Esophagitis, he or she will conduct a biopsy of the esophagus. The biopsy is usually done with a procedure called endoscopy, in which a small camera is inserted into the esophagus. Sometimes, the doctor will see rings of eosinophils (white blood cells) in the esophagus—but often it will appear normal. After the biopsy, the tissue will be inspected for evidence of eosinophils, If an EoE diagnosis is confirmed, the doctor will likely conduct allergy testing, looking for food and environmental allergies that contribute to the patient’s EoE. The International Gastrointestinal Eosinophil Researchers (TIGER) is currently in the process of evaluating and developing better clinical and diagnostic methods for determining food and aeroallergens that may be responsible for EoE.
Global Developmental Delay (GDD) is the general term used to describe a condition that occurs during the developmental period of a child between birth and 18 years. It is usually defined by the child being diagnosed with having a lower intellectual functioning than what is perceived as ‘normal’. It is usually accompanied by having significant limitations in communication. It is said to affect about 1-3% of the population.
WHAT ARE THE CAUSES OF GLOBAL DEVELOPMENTAL DELAY
The most common causes of GDD are chromosomal and/or genetic abnormalities such as Down’s Syndrome and Fragile X Syndrome or abnormalities with the structure or development of the brain or spinal cord such as Cerebral Palsy or Spina Bifida.
Other causes can include prematurity – being born too early – or infections, such as Congenital Rubella or Meningitis.
There are a number of diagnostic tests that can be done to identify the underlying cause of GDD. Sometimes these causes can be treated to cure the developmental delay, or at least to prevent it from worsening. However, often the cause is never able to be fully determined.
WHAT ARE THE SIGNS OF GLOBAL DEVELOPMENTAL DELAY?
The most common signs of GDD include:
- The child is unable to sit on the floor without support by 8 months;
- The child is unable to crawl by 12 months;
- The child has poor social skills/ judgment;
- The child is unable to roll over by 6 months;
- The child has communication problems
- The child has fine/ gross motor difficulties
- The child shows aggressive behavior as a coping skill
In some children, GDD is suspected soon after birth because of feeding difficulties or muscle-tone. In others, it is suspected later when learning or behavior difficulties occur at school.
IS THERE ANY TREATMENT FOR GLOBAL DEVELOPMENTAL DELAY?
There is no single treatment for GDD but there are ways to help some of the conditions that may be causing the delay. Once a pediatrician or neurologist has completed testing of the child, he/ she may advise on treatments for whatever underlying medical conditions that may exist. For example, hearing or visual impairment or therapeutic input by a Speech and Language Therapist, Occupational Therapist and Physiotherapist. It is possible that no cause will be found or that the cause that is identified may be difficult, if not impossible, to treat.
On the other hand, being aware of the conditions that are causing the delay can help parents, teachers and medical professionals to better counsel and guide children who are experiencing developmental problems.
www.cafamily.org.uk/medical-information/conditions/g/global-developmental-delay/
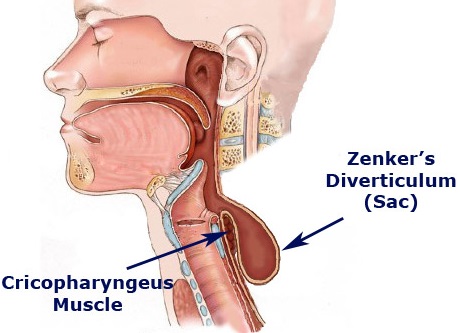
There are three types of esophageal diverticula, based on where they form: Zenker’s diverticula, the most common type, are in the upper esophagus, mid-thoracic diverticula in the mid-chest, and epiphanic diverticula just above the diaphragm. The diverticulum grows over time, so symptoms can gradually develop or worsen. One of the popular theories is that Zenker diverticulum may be caused by reflux.
However, there is absolutely no evidence to support this theory. Some patients with Zenker diverticulum who have been studied have evidence of reflux whereas others do not, and some have heartburn whereas others do not; there is no link between reflux disease and Zenker diverticulum even though I hear this misconception quite often. This supposed relationship is likely an extension of the concept that acid reflux can cause throat symptoms so that it is not just cough and laryngitis but also the sensation of fullness of the throat, Zenker diverticulum and other related symptoms.
Zenker diverticulum to Cricopharyngeal bar, a prominence of the upper esophageal sphincter seen on a radiograph, is a related condition that is often mentioned in radiology reports. Most of the time, it does not cause any symptoms and if a patient does not have dysphagia or a diverticulum, the condition can be ignored. The cricopharyngeal bar is occasionally seen in patients who complain of dysphagia. In this scenario, the same abnormalities of decreased opening described above in regard to Zenker diverticulum appear to occur in patients with cricopharyngeal bar. However, I cannot say that the two conditions are necessarily related, as there are no major or frequent reports of patients developing cricopharyngeal bar and then a diverticulum. Nevertheless, both conditions do appear to be in the same area and appear to share the same abnormality. Zenker diverticulum has been found, on barium swallow studies administered for indications other than dysphagia. However, the true prevalence of asymptomatic diverticula is unknown.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3104179/
Interesting read with photos
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3264939/
https://www.ajol.info/index.php/sajs/article/viewFile/80141/70404
If your child rocks or rhythmically moves part of her body just before or even during sleep, this may represent a condition called sleep-related rhythmic movement disorder (RMD). What is RMD? What conditions are associated with it and what similar disorders should be ruled out? Learn about rhythmic movement disorder, including treatment options to keep your child safe.
The Findings in Rhythmic Movement Disorder
Rhythmic movement disorder (RMD) may be observed in young children during the period just prior to or during sleep. During this period, an affected child may rock or move part of the body in a rhythmic manner. This may involve the arm, hand, head, or trunk. Other behaviors such as head banging or rolling may be observed. Although these movements may be relatively mild and constitute a form of self-soothing to ease into sleep, they can also be more extreme. More violent movements can occur and injuries may even result.
The condition is sometimes referred to as jactatio capitis nocturna or rhythmie du sommeil, which refer to the original descriptions of the condition from 1905.
When Does Rhythmic Movement Disorder Occur?
https://www.verywell.com/what-is-sleep-related-rhythmic-movement-disorder-3014802
Keywords: mitomycin C, esophageal stricture, tracheoesophageal fistula
Esophageal stricture is a well-described complication following tracheoesophageal fistula repair. Herein, we report two patients who had persistent esophageal strictures after several months of repeat balloon dilatations. Each patient was treated with a single application of topical mitomycin C in addition to esophageal dilatation, which resulted in complete resolution of the stricture.
Tracheoesophageal fistulas (TEFs) are treated with operative ligation of the distal fistula and anastomosis of the esophageal limbs. The development of an esophageal stricture at the anastomosis is a well-described complication after repair, occurring in approximately one-third of patients. Endoscopic balloon dilatation is currently the preferred method of treatment for both initial and recurrent esophageal strictures. Recently, the use of topical mitomycin C (MMC) for the treatment of esophageal strictures after surgical repair and caustic injury has been reported. In these studies, MMC treatment was largely successful in the resolution of esophageal strictures with a minimal complication profile. However, the vast majority of the reported cases of MMC use in esophageal strictures have been secondary to caustic injury, with a paucity of reported cases after newborn TEF repair. Herein, we report the successful use of topical MMC in combination with balloon dilatation for the treatment of refractory esophageal strictures following TEF repair in two neonates with complete resolution of the stricture without complications.
Case 1
(a) Esophagram of a tight stricture (white arrow) at the anastomosis in a 39-week-old infant girl, born with VACTERL syndrome. (b) Esophagram showing resolution (white arrow) of the esophageal stricture after balloon dilatation and treatment with mitomycin C.
A 39-week infant girl, born with VACTERL syndrome, was initially diagnosed with a long-gap pure esophageal atresia due to inability to pass a nasogastric tube and a gasless abdomen on abdominal radiograph. Shortly after birth, the patient underwent a gastrostomy tube placement and end colostomy for imperforate anus. Two months later, she underwent a right-sided thoracotomy, where a Type C TEF was encountered, as opposed to a pure esophageal atresia. She underwent ligation of the distal fistula, resection of a nonpatent fibrous cord of the distal esophageal limb with subsequent anastomosis of the esophageal limbs under tension. A postoperative esophagram revealed an anastomotic leak, which eventually resolved. Four months postoperatively, she underwent a follow-up esophagram which revealed a tight stricture at the anastomosis. She underwent endoscopic balloon dilatation with fluoroscopic guidance every month for four consecutive months with persistence of the stricture at the anastomosis and no improvement.
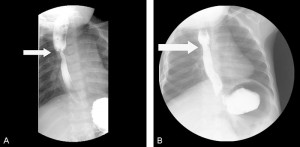
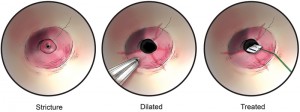
At 10 months of age, the patient underwent a fourth endoscopic and fluoroscopic guided balloon dilatation of the stricture as before. After adequately dilating the stricture and visualizing circumferential, superficial linear disruption of the strictured ring at the anastomosis, we soaked 1/2-inch × 1/2-inch cottonoids in MMC solution (0.4 mg/mL). A rigid esophagoscope was utilized to apply the MMC-soaked cottonoid onto the left side of the stricture for a 1-minute time period followed by the placement of another MMC-soaked cottonoid on the right side of the stricture for 1 minute (fig-2). The cottonoids were removed, and the esophagus appeared intact. Follow-up surveillance endoscopy was performed after MMC application, which showed no evidence of an anastomotic stricture (fig-1b). No additional balloon dilatation was required thereafter, and no recurrence of her stricture has since occurred. Now at 3 years of age, she remains asymptomatic, eats regular food after gastrostomy tube removal, and has not required further treatment.
Case 2
A 31-week twin infant girl underwent repair of a Type C TEF. Before thoracotomy, the patient’s clinical condition deteriorated with marked distention of her abdomen. An open gastrostomy was first performed and placed on water seal, and a thoracotomy with ligation of a distal TEF and esophagus-oesophagostomy was performed under moderate tension. One month later, she developed symptoms of feeding intolerance, reflux, and tracheal aspiration. A repeat esophagram demonstrated a near-obstructing stricture in the mid esophagus. The stricture was short, circumferential, and located at the site of the anastomosis. She was taken to the operating room for endoscopic balloon dilatation with fluoroscopic guidance, and subsequently underwent three more monthly balloon dilatations; however, there was no interval improvement in the stricture.
At 5 months of age, MMC was applied during the fifth esophageal dilatation in the same manner as described above. The patient underwent a follow-up surveillance endoscopy, which demonstrated no evidence of residual stricture. Two and a half years after the sole MMC application and dilatation, she remains asymptomatic.
Discussion, there is more to read and more photos etc click on the link below.
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4335951/?log$=activity
2014 Jan
A case of VACTERL and non-VACTERL association without the “V and L”.
VACTERL is a cluster of congenital malformations based on the non-random association of various congenital malformations in a single patient. Here “V” denotes vertebral defects or vascular anomalies (single umbilical artery), “A” anal atresia, “C” cardiac abnormalities, “TE” tracheoesophageal fistula, “R”renal (kidney) abnormalities and “L” for limb anomalies) It is called an association, rather than a syndrome because the complications are not pathogenetically related, tend to occur more frequently than expected and are thought to be linked to embryonic mesodermal defects. Studies have reported the coexistence of various other congenital malformations such as respiratory, cerebral anomalies, which are frequently referred as non-VACTERL-type of associations. Diagnosis of VACTERL association is done only when at least three of the above mentioned congenital malformations are identified in a patient. Although 80% of these cases have vertebral defects, our case is unique as the patient does not have one of the commonest occurring association i.e., vertebral anomalies, but has all other associations and an additional non VACTERL brain anomaly, hitherto unreported in the literature. The other highlight of this case is although reports say that VACTERL babies with the ipsilateral renal disorder have the same side limb defects, our case has a renal anomaly with no limb anomaly. Finally, VACTERL and non VACTERL association were considered in our patient in view of ventricular septal defect, tracheoesophageal fistula, anal atresia, renal anomaly, seizure disorder and global developmental delay due to pontocerebellar hypoplasia.
KEYWORDS:
Crossed fused ectopic kidney; VSD; anal atresia; gastroesophageal reflux scintigraphy; methylene diphosphonate bone scan; renal and limb anomalies; tracheoesophageal fistula; vascular anomalies; vertebral defects
