Flourish Pediatric Esophageal Atresia Device
How did this device come to be I hear you ask? it came from a Dr. Zaritzky
(originator of this idea)
and it is named
Flourish
What is this idea?
How did this device come to be I hear you ask? it came from a Dr. Zaritzky
(originator of this idea)
and it is named
What is this idea?
This idea has been around since 2016
But finding the right company to make and understand how it then works takes time, then you need to get it passed and in doing so the product then receives the IRB standard, passed by the USA medical board to allow it to move forwards.
So what is this idea?
HUMANITARIAN DEVICE
Authorized by federal law for use in the treatment of lengthening atretic esophageal ends and creating an anastomosis with a non-surgical procedure in pediatric patients, up to one year of age with esophageal atresia without tracheoesophageal fistula (TEF), or in pediatric patients up to one year of age for whom a concurrent TEF has been closed as a result of a prior procedure. The effectiveness of this use has not been demonstrated.
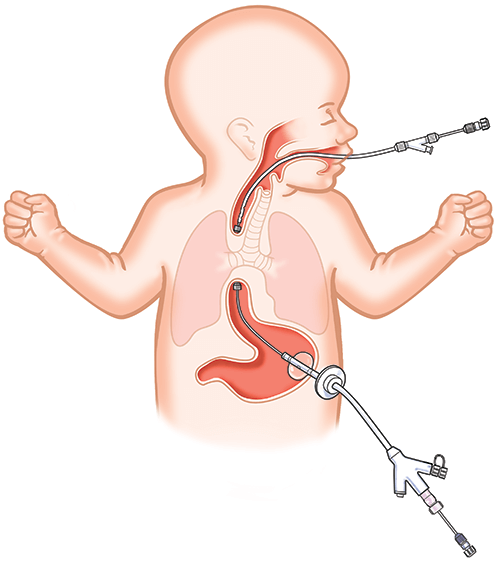
The Flourish™ Device consists of two (2) catheters. Each catheter has a magnet at its tip. The oral catheter passes
through the mouth and into the esophagus so that the magnet reaches the bottom of the upper esophageal pouch.
The gastric catheter replaces the current gastric feeding tube which is removed. The gastric catheter passes into the
stomach through the existing gastrostomy site and upward through the stomach so that the magnet on the far end
reaches the top of the gastric esophageal pouch.
How does the device work?
The magnets placed at each end of the esophagus attract each other This causes the ends of the esophagus
to stretch toward each other Successful connection of the tissue of the upper and lower esophageal pouches are
confirmed with x-ray imaging The surrounding tissues will grow together while the tissue trapped between the
magnets will necrose and slough away. This creates an open passage from the mouth to the stomach that typically occurs
in 3 to 13 days.
There is a leading USA team of Tef Surgeons, who are looking into trying this device out, if the Parents give their consent then a study can be run.
Remember this is a NEW idea
The company we found doing this is called, Cook medical. They have a device called Flourish. This is a humanitarian use device that is available for atresia in babies under one year of age and a gap less than 4cm, with no TEF or with repairs I also like to learn from parents that have dealt with EA since I work directly with surgeons and their families. We have worked with various surgeons in the US and Canada.
They need info to make sure they are able to offer this device as an alternative for EA before surgery. If you would like to know more please let me know and we can schedule a call.
This will allow parents that have a baby with EA to look into a less invasive treatment option.
They are looking into working with a leading team of Surgeons in the USA. This will be his first Flourish procedure. At this moment they are rolling this technology out slowly because of the status of the device. In the US it is a HUD (Humanitarian Use Device), this requires IRB (institutional review board) approval to be used in a hospital. Outside of the US, this device falls under Compassionate Use which requires approval from the regulatory body of the country requesting the device.
Responsive website designed & developed by
![]()